Addressing patient access with off-the-shelf CAR-T cell therapy
CAR-T cell therapies are engineered to recognize and kill disease-causing cells based on specific markers on the target cell surface. CAR-T cell therapies manufactured from a patient's T cells, also known as autologous CAR-T cells, have been approved by the U.S. Food and Drug Administration (FDA) due to their effectiveness for targeting and destroying blood cancers. However, the bespoke nature of an individualized therapy means it is challenging to scale product availability to large patient populations. Some of the challenges include limited patient access, long wait times, frequent need for interim therapies, long manufacturing timelines, and the risk of product manufacturing failure. To address these challenges, we are developing allogeneic CAR-T cell therapies derived from healthy donor T cells that are genome-edited and manufactured in advance to be readily available off-the-shelf with the potential to reach broader numbers of patients with hematologic malignancies.
Armoring for potentially improved activity
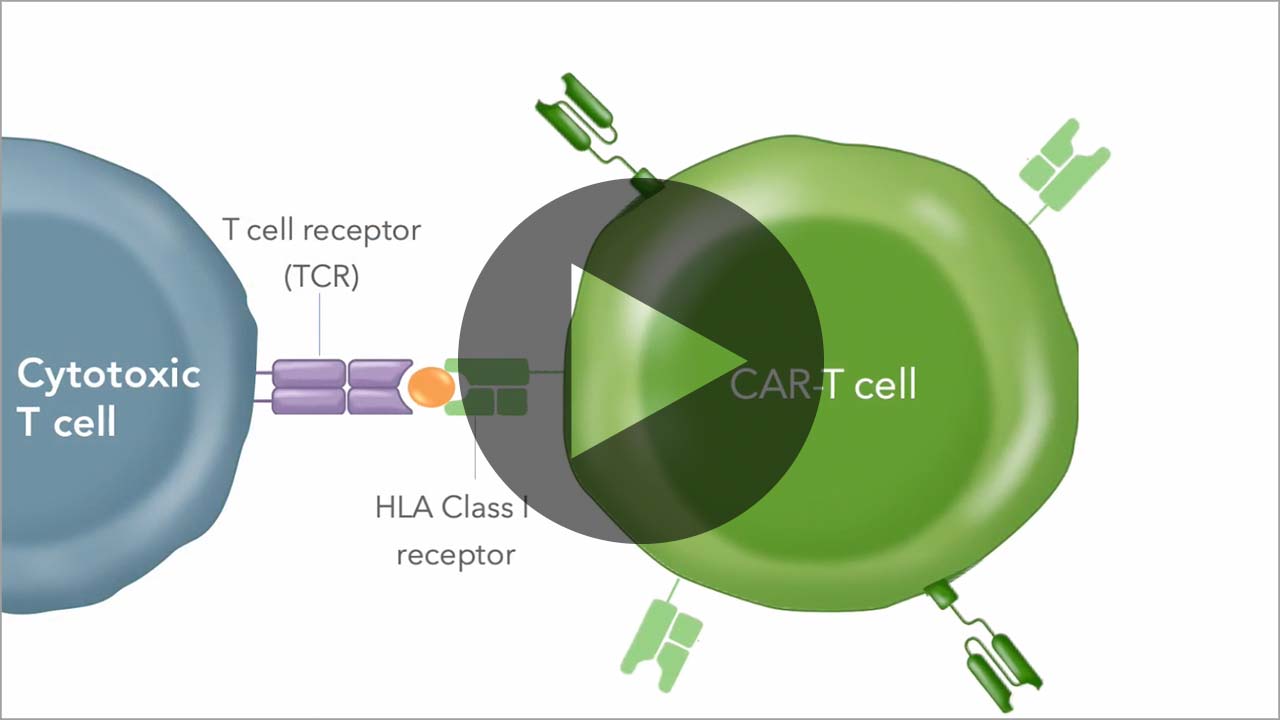
Allogeneic CAR-T cells must be armored to deliver sufficient activity prior to being rejected as "foreign" by the patient's immune system. We use our chRDNA genome-editing technology to implement multiple different armoring strategies to unlock the full potential of our allogeneic CAR-T cell therapies.
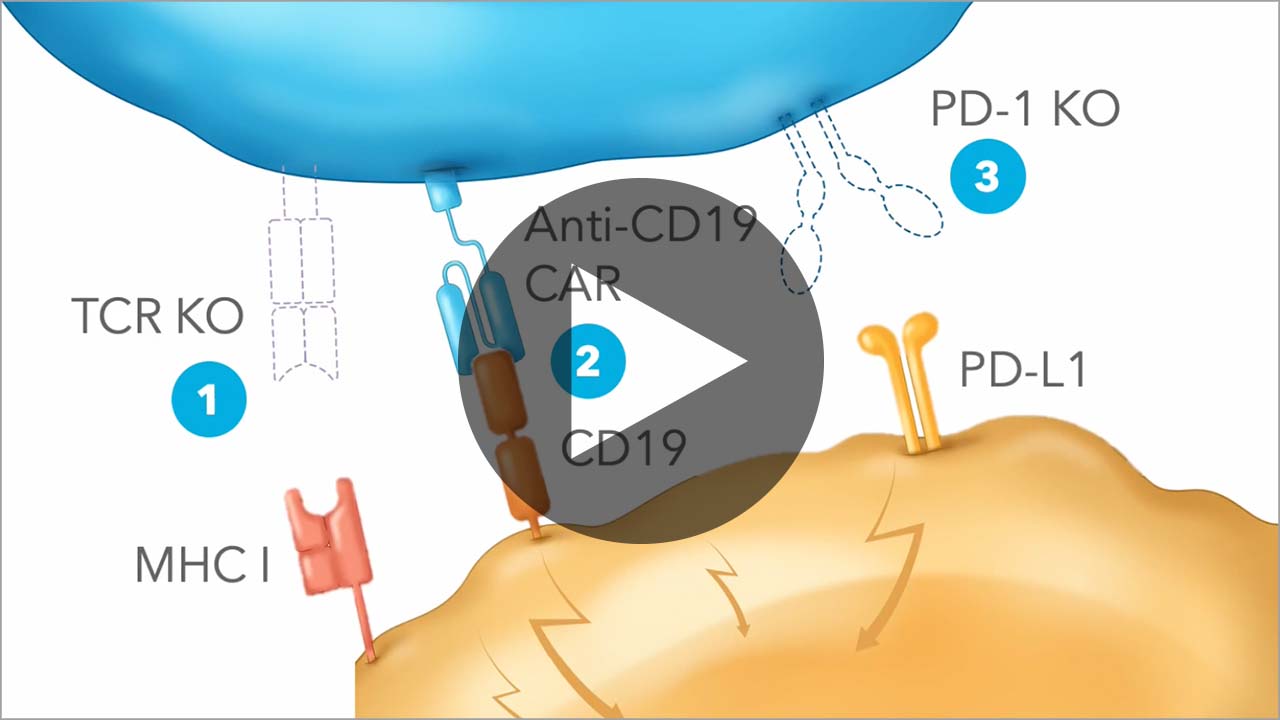
CB-010 is armored with checkpoint disruption to reduce T cell exhaustion
CB-010, an allogeneic anti-CD19 CAR-T cell therapy, has three edits engineered to target disease-causing CD19-positive B cells, mitigate risk of graft versus host disease (GvHD), and reduce T cell exhaustion.
CB-010 is being evaluated in the ANTLER Phase 1 clinical trial for adults with relapsed or refractory B cell non-Hodgkin lymphoma (B-NHL). Learn more about our ongoing clinical trials here.
CB-011 armored with immune cloaking to prevent both T and NK cell-mediated immune rejection
CB-011, an allogeneic anti-BCMA CAR-T cell therapy, has four edits engineered to target BCMA-positive cancer cells, mitigate risk of GvHD, and reduce immune rejection by both T cells and natural killer (NK) cells.
CB-011 is being evaluated in the CaMMouflage Phase 1 clinical trial for adults with relapsed or refractory multiple myeloma. Learn more about our ongoing clinical trials here.